Utilizing Maze Compass™, we are harnessing the power of human genetics to develop novel, small molecule precision medicines for patients living with renal, cardiovascular and related metabolic diseases, including obesity.
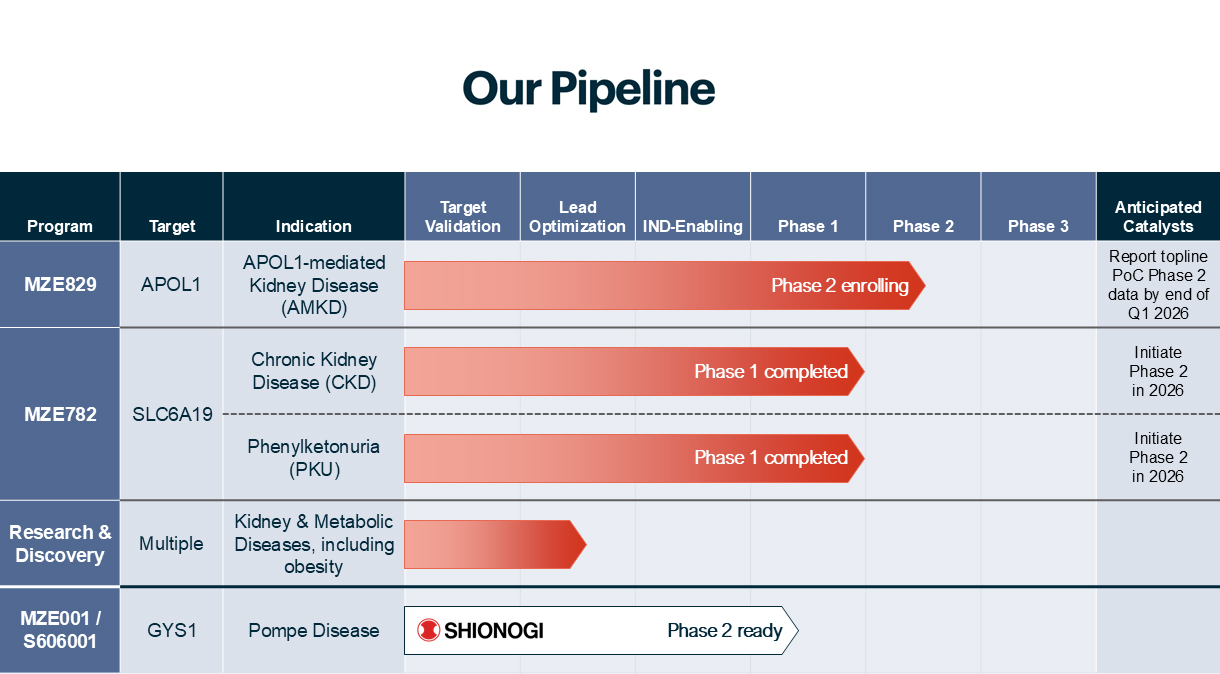
Maze APOL1 Program
APOL1-Mediated Kidney Disease
In the United States, approximately six million, or 13%, of African Americans have mutations of both copies of the high-risk APOL1 gene variants, and are at risk for developing AKD. It is currently estimated that approximately 20%, or over one million, of those individuals have AKD.
Click here to learn more
Maze Precision Renal Program
Chronic Kidney Disease
Chronic kidney disease impacts approximately 37 million, or 1 in 7 individuals in the U.S. alone2. Current treatments for chronic kidney disease do not address the underlying cause and instead focus on slowing the progression.
Click here to learn more
2. Source: https://www.cdc.gov/kidneydisease/publications-resources/ckd-national-facts.html